|
Enantiomerically
Pure Aziridine-2-carboxylates and Their Derivatives for Organic Synthesis
 Preparation of Enantiomerically Pure Aziridine-2-carboxylates
Preparation of Enantiomerically Pure Aziridine-2-carboxylates
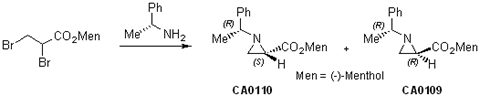
 Transformation of the Side Chain Attached to the Chiral Aziridine
Transformation of the Side Chain Attached to the Chiral Aziridine
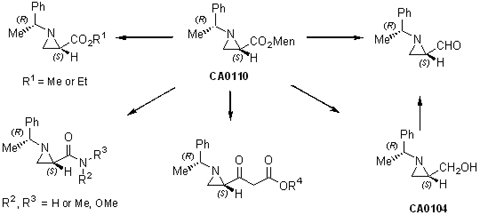
 Preparation of 2-acylaziridines
Preparation of 2-acylaziridines
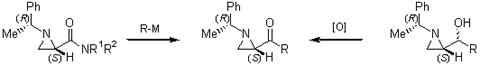
 Stereoselective reduction of 2-acylaziridines to provide vicinal amino
alcohols
Stereoselective reduction of 2-acylaziridines to provide vicinal amino
alcohols
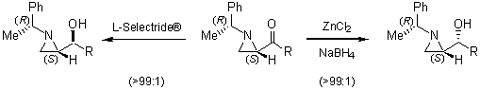
 Stereoselective additions to the aldimine
Stereoselective additions to the aldimine
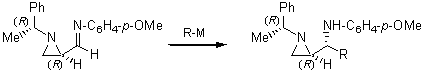
 Preparation of 2-vinylaziridines
Preparation of 2-vinylaziridines

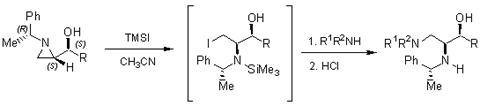
 Aziridine Ring-opening
Aziridine Ring-opening
 Regioselective reductive ring-opening
Regioselective reductive ring-opening
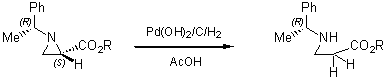
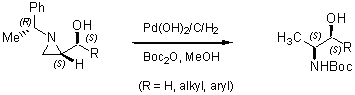
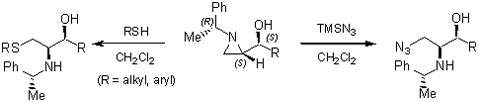
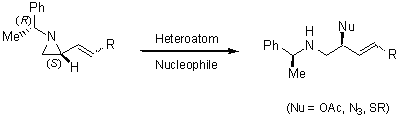
 Regioselective ring-opening with heteroatom nucleophiles
Regioselective ring-opening with heteroatom nucleophiles
- with oxygen
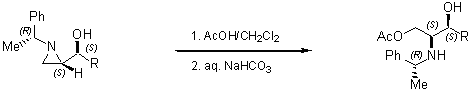
- with nitrogen and sulfur
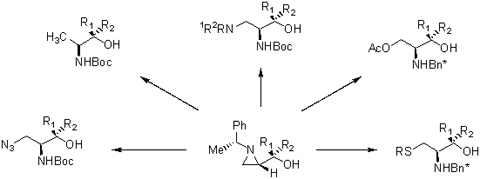
 Summary
Summary
 Rearrangements and ring expansions
Rearrangements and ring expansions
 Preparation of 5-functionalized oxazolidinones
Preparation of 5-functionalized oxazolidinones
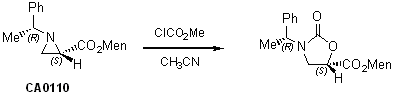
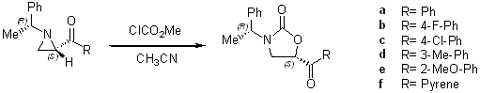
 Preparation of 4-functionalized oxazolidinones
Preparation of 4-functionalized oxazolidinones
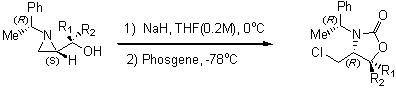
 Asymmetric synthesis of amino acid derivatives
from chiral aziridines
Asymmetric synthesis of amino acid derivatives
from chiral aziridines
 Representative examples
Representative examples
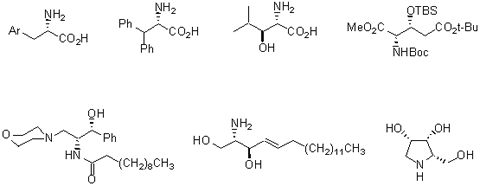
Both
(2R)- and (2S)-aziridine-2-carboxylates and their derivatives are now
commercially available in bulk quantities in optically pure forms. Stereo-
and regioselective transformations including aziridine ring-opening reactions
enable us to prepare a variety of nitrogen-containing molecules. Some
of them may be useful in the practical synthesis of commercially valuable
compounds and as starting molecules to generate diverse compound libraries.
|
